Within FAIRVASC Workpackage 2 (WP2) will capture the legal and ethical environment of each registry, patient perspectives on data sharing and re-use, and also the national and international legislation and regulations that apply to FAIRVASC. D2.3 is the final in a series of deliverable describing information governance compliance across the FAIRVASC registries.
You may also like
FAIRVASC-focused meeting was held in Barcelona during the 21st International Vasculitis Workshop on 9th of April 2024. FAIRVASC Session 12:00 Welcome and Introduction […]
Application for a clinical RMD research methodology was approved by the EULAR research Committee on the 12th of MARCH 2024, for a total […]
The FAIRVASC project held its first (kick-off) meeting over 2 days (03rd and 09th June 2020). All partners were represented, and the meeting was […]
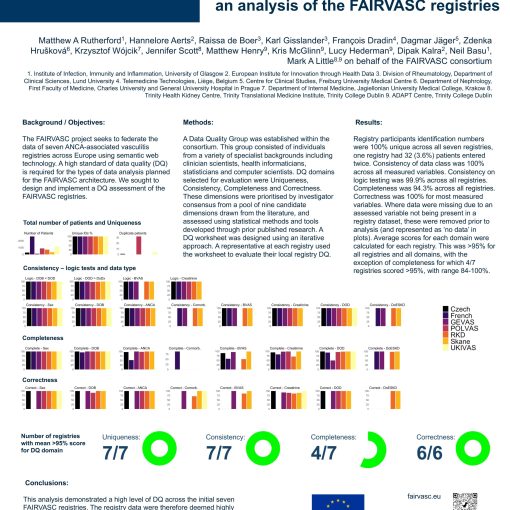
We are happy to present an analysis of the data quality from the FAIRVASC registries. The Data Quality Team on behalf of […]